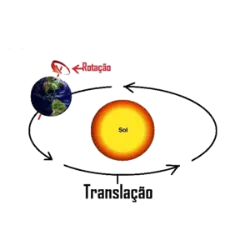
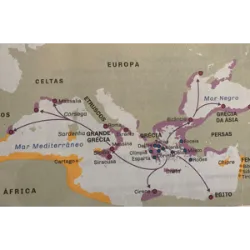
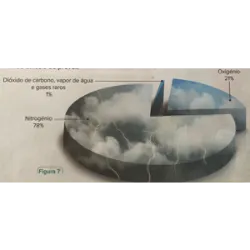
Curiosities, games, challenges and quiz on various topics
Atoms are made up of three fundamental particles: Protons, Neutrons and Electrons. The nucleus accounts for most of the atomic mass and the electron cloud determines the size of the atom. Atoms can acquire or lose electrons, forming ions with an electrical charge, such as anions (which gain electrons) and cations (which lose electrons). The mass of the atom is concentrated in its nucleus, as the mass of protons and neutrons is the same. Isotopes are atoms of the same chemical element, so they have the same atomic number but different mass number due to the fact that they have different numbers of neutrons.