Here you will master your favorite subject, enter the
Start by checking out some of the fun facts and then explore the challenges
 July 9th marks the Constitutional Revolution of 1932, a movement that represented the struggle of the people of São Paulo for a new Constitution for Brazil. The uprising was organized by the Partido Republicano Paulista (PRP), with support from the Democratic Party, and included the participation of both civilian and military forces from São Paulo, as well as support from the states of Mato Grosso and Rio Grande do Sul. The trigger was the death of four young men during a protest against the government of Getúlio Vargas, which led to the creation of the MMDC movement. Although the São Paulo troops were defeated in October 1932, the mobilization resulted in the convening of the Constituent Assembly in 1933 and the enactment of the 1934 Constitution. July 9th was established as a state holiday in 1997, after the approval of Bill No. 710/1995, and is exclusive to São Paulo. The date honors the struggle for democracy and is remembered as a milestone in the history of the state.
July 9th marks the Constitutional Revolution of 1932, a movement that represented the struggle of the people of São Paulo for a new Constitution for Brazil. The uprising was organized by the Partido Republicano Paulista (PRP), with support from the Democratic Party, and included the participation of both civilian and military forces from São Paulo, as well as support from the states of Mato Grosso and Rio Grande do Sul. The trigger was the death of four young men during a protest against the government of Getúlio Vargas, which led to the creation of the MMDC movement. Although the São Paulo troops were defeated in October 1932, the mobilization resulted in the convening of the Constituent Assembly in 1933 and the enactment of the 1934 Constitution. July 9th was established as a state holiday in 1997, after the approval of Bill No. 710/1995, and is exclusive to São Paulo. The date honors the struggle for democracy and is remembered as a milestone in the history of the state.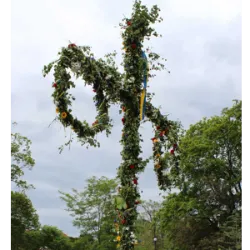 The June festivals in Sweden, known as Midsommarafton, are considered the most important national celebrations in the country, surpassing even Christmas in popular participation. Held between June 20 and 26, Friday is the traditional day for the celebration, marked by several typical traditions.
The June festivals in Sweden, known as Midsommarafton, are considered the most important national celebrations in the country, surpassing even Christmas in popular participation. Held between June 20 and 26, Friday is the traditional day for the celebration, marked by several typical traditions.One of the central symbols of the festival is the majstången — a maypole decorated with flowers and leaves, erected in the center of the villages, around which participants dance in a circle. This tradition has common roots with the Maypole of the Germanic peoples and is linked to fertility and the renewal of nature.
During Midsommarafton, people dress in rural clothes, sing traditional carols and celebrate with typical seasonal foods, such as strawberries and potatoes. Charms are also part of the festival, especially among young women, who make bouquets with seven or nine flowers to dream about their future husbands.
In addition, popular belief says that the herbs harvested at this time have special powers and that the water from the springs brings health. Houses are decorated with flowers and leaves to attract luck. Big cities like Stockholm and Gothenburg are almost empty, as Swedes take refuge in their summer homes to celebrate the date. The traditional balloons of the festival, however, often cause accidents.
 The big news for the 2025 edition of the Club World Cup will be the presence of Inter Miami CF, a club from the United States, which has secured a spot as the host country's representative. The North American team, which gained notoriety with the arrival of star Lionel Messi in 2023, secured its qualification by winning the 2024 MLS Supporters' Shield, an award given to the team with the best record in the regular season of Major League Soccer (MLS).
The big news for the 2025 edition of the Club World Cup will be the presence of Inter Miami CF, a club from the United States, which has secured a spot as the host country's representative. The North American team, which gained notoriety with the arrival of star Lionel Messi in 2023, secured its qualification by winning the 2024 MLS Supporters' Shield, an award given to the team with the best record in the regular season of Major League Soccer (MLS).This spot, a first for the club and exclusive to the team from the host country, marks an important participation for soccer in the United States, which is on the rise with the increase in interest in big international stars and the growth of domestic competitions. In addition, the presence of Inter Miami CF in the tournament puts North American soccer in the spotlight, attracting even more audiences and sponsors to the sport in the country.
Inter Miami's qualification also represents the importance of a competitive championship like MLS, which is beginning to consolidate itself as a relevant league on the global stage. The club will now have the chance to face some of the best teams in the world at the 2025 Club World Cup, with the hope of making its mark on the tournament's history.
 To choose the perfect beer, try different styles and brands, take food pairings into account and consider flavors, acidity and alcohol content. For example, for pasta, choose Dark Lager, Strong Ale or Pilsner, while for barbecue, opt for Pilsner, Stout or Brown Ale. Seafood pairs well with English Pale Ale, Amber Ale, Hefeweizen and Witbier, and for desserts, choose Stout, Porter or Fruit Lambic. Cheeses can be paired with a variety of beers, but dark beers like porter and stout generally work well. If it is vegetarian food, Pilsner is a good choice due to the variety of vegetarian ingredients.
To choose the perfect beer, try different styles and brands, take food pairings into account and consider flavors, acidity and alcohol content. For example, for pasta, choose Dark Lager, Strong Ale or Pilsner, while for barbecue, opt for Pilsner, Stout or Brown Ale. Seafood pairs well with English Pale Ale, Amber Ale, Hefeweizen and Witbier, and for desserts, choose Stout, Porter or Fruit Lambic. Cheeses can be paired with a variety of beers, but dark beers like porter and stout generally work well. If it is vegetarian food, Pilsner is a good choice due to the variety of vegetarian ingredients.On the Syndicato blog, you can find more tips on ideal combinations.
 Opened in 1978, the Volta Redonda Municipal Zoo has established itself as one of the largest and most important spaces for environmental preservation and leisure in the state of Rio de Janeiro. Located in the Vila Santa Cecília neighborhood, the site was created with the aim of providing contact with nature in the middle of an industrial city, offering environmental education to the population and protecting wildlife.
Opened in 1978, the Volta Redonda Municipal Zoo has established itself as one of the largest and most important spaces for environmental preservation and leisure in the state of Rio de Janeiro. Located in the Vila Santa Cecília neighborhood, the site was created with the aim of providing contact with nature in the middle of an industrial city, offering environmental education to the population and protecting wildlife.With more than 300 animals of different species — including birds, mammals and reptiles — the zoo has become a regional reference in veterinary care and conservation programs. Highlights include the immersion aviary and the themed spaces aimed at educating children about the importance of biodiversity and the environment.
In addition to its educational function, the zoo is one of the most visited tourist attractions in the city, receiving thousands of visitors each year. The space has trails, picnic areas and playgrounds, integrating leisure and environmental awareness.
Over the years, the Volta Redonda Zoo has undergone renovations and structural improvements, reaffirming its role not only as a tourist destination, but also as a symbol of the relationship between urban development and environmental sustainability.
Choose your favorite theme
 CLUB WORLD CUP
CLUB WORLD CUP Academic
Academic Stranger Things
Stranger Things The Walking Dead
The Walking Dead Sports
Sports Harry Potter
Harry Potter Beers
Beers Music
Music Games
Games Copa Libertadores
Copa Libertadores Cinema and TV
Cinema and TV Tourism
Tourism FIFA Women's Cup
FIFA Women's Cup Gymnastics
Gymnastics Rock n Roll
Rock n Roll KPOP
KPOP Tennis
Tennis History
History BasketBall
BasketBall Board games
Board games RPG
RPG Math
Math Barbie
Barbie Roblox
Roblox Europe Tourism
Europe Tourism Card games
Card games Portuguese Language
Portuguese Language Samba & Pagode
Samba & Pagode Brazilian soap operas
Brazilian soap operas North America Tourism
North America Tourism Hip Hop
Hip Hop Astrology
Astrology Natural Sciences
Natural Sciences MarioBros
MarioBros Athletics
Athletics MPB
MPB Geography
Geography TV Séries
TV Séries VolleyBall
VolleyBall Electronic games
Electronic games Sertanejo
Sertanejo FortNite
FortNite Portugal
Portugal Funk
Funk Swimming
Swimming English Language
English Language South America Tourism
South America Tourism Pop Music
Pop Music Surf
Surf Animations
Animations Olympics
Olympics Soccer
Soccer Movies
Movies Physics-Chemistry
Physics-Chemistry Forró
Forró Portuguese Folklore
Portuguese Folklore MineCraft
MineCraft Brazil Tourism
Brazil Tourism Magic the Gathering
Magic the Gathering Portugal Tourism
Portugal Tourism France Tourism
France Tourism Chile Tourism
Chile Tourism Paraguay Tourism
Paraguay Tourism Canada Tourism
Canada Tourism Aruba Tourism
Aruba Tourism Brazilian Socccer
Brazilian Socccer Brasileirão
Brasileirão Spain Tourism
Spain Tourism Yoga
Yoga United Kingdom Tourism
United Kingdom Tourism Bonaire Tourism
Bonaire Tourism Suriname Tourism
Suriname Tourism French Guiana
French Guiana Colombia Tourism
Colombia Tourism NBA
NBA Diversity
Diversity Uruguay
Uruguay Peru Tourism
Peru Tourism Mexico Tourism
Mexico Tourism Guyana Tourism
Guyana Tourism Anime
Anime Portuguese Wines
Portuguese Wines BlockBusters
BlockBusters Argentina Tourism
Argentina Tourism Spanish Socccer
Spanish Socccer Falkland Island Tourism
Falkland Island Tourism Portuguese Socccer
Portuguese Socccer Bolivia Tourism
Bolivia Tourism United States Tourism
United States Tourism Animated Cartoon
Animated Cartoon Italy Tourism
Italy Tourism Venezuela Tourism
Venezuela Tourism Curaçao Tourism
Curaçao Tourism French Cuisine
French Cuisine Demon Slayer
Demon Slayer Athletico Paranaense
Athletico Paranaense Brazilian Folklore
Brazilian Folklore Prehistory
PrehistoryYou know? Today is the day of..
 World Allergy Day is celebrated on July 8th every year. This date is an initiative by the World Allergy Organization (WAO) to increase awareness about allergies and improve the diagnosis, treatment and prevention of these conditions. Allergies can affect people of all ages and include a variety of conditions, such as food allergies, seasonal allergies (such as hay fever), insect sting allergies, drug allergies, and pet allergies, among others. On World Allergy Day, activities are held around the world to educate the public about allergies, their symptoms and treatments, as well as to promote scientific research into allergies and immunology.
World Allergy Day is celebrated on July 8th every year. This date is an initiative by the World Allergy Organization (WAO) to increase awareness about allergies and improve the diagnosis, treatment and prevention of these conditions. Allergies can affect people of all ages and include a variety of conditions, such as food allergies, seasonal allergies (such as hay fever), insect sting allergies, drug allergies, and pet allergies, among others. On World Allergy Day, activities are held around the world to educate the public about allergies, their symptoms and treatments, as well as to promote scientific research into allergies and immunology.Check out other curiosities and quiz topics

July 9th marks the Constitutional Revolution of 1932, a movement that represented the struggle of the people of São Paulo for a new Constitution for Brazil. The uprising was organized by the Partido Republicano Paulista (PRP), with support from the Democratic Party, and included the participation of both civilian and military forces from São Paulo, as well as support from the states of Mato Grosso and Rio Grande do Sul. The trigger was the death of four young men during a protest against the government of Getúlio Vargas, which led to the creation of the MMDC movement. Although the São Paulo troops were defeated in October 1932, the mobilization resulted in the convening of the Constituent Assembly in 1933 and the enactment of the 1934 Constitution. July 9th was established as a state holiday in 1997, after the approval of Bill No. 710/1995, and is exclusive to São Paulo. The date honors the struggle for democracy and is remembered as a milestone in the history of the state.
The June festivals in Sweden, known as Midsommarafton, are considered the most important national celebrations in the country, surpassing even Christmas in popular participation. Held between June 20 and 26, Friday is the traditional day for the celebration, marked by several typical traditions.
One of the central symbols of the festival is the majstången — a maypole decorated with flowers and leaves, erected in the center of the villages, around which participants dance in a circle. This tradition has common roots with the Maypole of the Germanic peoples and is linked to fertility and the renewal of nature.
During Midsommarafton, people dress in rural clothes, sing traditional carols and celebrate with typical seasonal foods, such as strawberries and potatoes. Charms are also part of the festival, especially among young women, who make bouquets with seven or nine flowers to dream about their future husbands.
In addition, popular belief says that the herbs harvested at this time have special powers and that the water from the springs brings health. Houses are decorated with flowers and leaves to attract luck. Big cities like Stockholm and Gothenburg are almost empty, as Swedes take refuge in their summer homes to celebrate the date. The traditional balloons of the festival, however, often cause accidents.

The big news for the 2025 edition of the Club World Cup will be the presence of Inter Miami CF, a club from the United States, which has secured a spot as the host country's representative. The North American team, which gained notoriety with the arrival of star Lionel Messi in 2023, secured its qualification by winning the 2024 MLS Supporters' Shield, an award given to the team with the best record in the regular season of Major League Soccer (MLS).
This spot, a first for the club and exclusive to the team from the host country, marks an important participation for soccer in the United States, which is on the rise with the increase in interest in big international stars and the growth of domestic competitions. In addition, the presence of Inter Miami CF in the tournament puts North American soccer in the spotlight, attracting even more audiences and sponsors to the sport in the country.
Inter Miami's qualification also represents the importance of a competitive championship like MLS, which is beginning to consolidate itself as a relevant league on the global stage. The club will now have the chance to face some of the best teams in the world at the 2025 Club World Cup, with the hope of making its mark on the tournament's history.

To choose the perfect beer, try different styles and brands, take food pairings into account and consider flavors, acidity and alcohol content. For example, for pasta, choose Dark Lager, Strong Ale or Pilsner, while for barbecue, opt for Pilsner, Stout or Brown Ale. Seafood pairs well with English Pale Ale, Amber Ale, Hefeweizen and Witbier, and for desserts, choose Stout, Porter or Fruit Lambic. Cheeses can be paired with a variety of beers, but dark beers like porter and stout generally work well. If it is vegetarian food, Pilsner is a good choice due to the variety of vegetarian ingredients.
On the Syndicato blog, you can find more tips on ideal combinations.

Opened in 1978, the Volta Redonda Municipal Zoo has established itself as one of the largest and most important spaces for environmental preservation and leisure in the state of Rio de Janeiro. Located in the Vila Santa Cecília neighborhood, the site was created with the aim of providing contact with nature in the middle of an industrial city, offering environmental education to the population and protecting wildlife.
With more than 300 animals of different species — including birds, mammals and reptiles — the zoo has become a regional reference in veterinary care and conservation programs. Highlights include the immersion aviary and the themed spaces aimed at educating children about the importance of biodiversity and the environment.
In addition to its educational function, the zoo is one of the most visited tourist attractions in the city, receiving thousands of visitors each year. The space has trails, picnic areas and playgrounds, integrating leisure and environmental awareness.
Over the years, the Volta Redonda Zoo has undergone renovations and structural improvements, reaffirming its role not only as a tourist destination, but also as a symbol of the relationship between urban development and environmental sustainability.

Vila Nova de Paiva is a true centre of traditional craftsmanship, where ancient techniques remain alive and well, adapted to the times. The local culture is marked by a rich legacy of knowledge passed down from generation to generation, with particular emphasis on:
The production of linen fabrics, once a pillar of the local economy, was gradually replaced by the cotton industry, but artisanal production techniques are still preserved, such as burel, woven at home, especially with the help of the Fráguas treadmill, which improved the finish. The region is also known for the production of wool blankets and burel clothing, typical products of Vila Nova de Paiva and Pendilhe.
Ceramics, tiles and wrought iron are still practiced, with emphasis on restoration work and figurative sculptures in granite. Basketwork and clog-making are other traditional crafts in Pendilhe, Alhais, Queiriga and Vila Nova de Paiva, in addition to the production of wooden miniatures of agricultural implements, especially in Vila Cova-à-Coelheira.
Stonework, granite and wood sculpture, and the art of lace are other expressions of great importance in the region, keeping local history and culture alive.

Realism / Naturalism / Parnassianism (19th century)
Machado de Assis (Dom Casmurro) – Brazil
Eça de Queirós (Cousin Basil) – Portugal
Gustave Flaubert (Madame Bovary) – France
Lima Barreto (The Sad End of Policarpo Quaresma) – Brazil
Olavo Bilac – Parnassian poetry – Brazil
Émile Zola – French naturalism
Modernism (20th century – until 1945)
James Joyce (Ulysses) – Ireland
Virginia Woolf (Mrs. Dalloway) – England
Franz Kafka (The Metamorphosis) – Czechoslovakia
Mário de Andrade (Macunaíma) – Brazil
Oswald de Andrade (Anthropophagic Manifesto) – Brazil
Carlos Drummond de Andrade – Modern poetry – Brazil
Contemporary (post-1945 to present)
Clarice Lispector (The Hour of the Star) – Brazil
Gabriel García Márquez (One Hundred Years of Solitude) – Colombia
José Saramago (Blindness) – Portugal
Chimamanda Ngozi Adichie – Nigeria, post-colonial literature
Haruki Murakami – Japan, modern surrealism
Conceição Evaristo – Brazil, Afro-Brazilian literature
Margaret Atwood (The Handmaid's Tale) – Canada

Inversion is when the normal order of words in a sentence is changed, usually for emphasis, style, or to create a specific grammatical structure.
- Normally, the order in English is:
Subject + Verb + Complement
Example: She had never seen such a thing.
With inversion, we change this:
- Negative adverbials
These expressions force an inversion when placed at the beginning of the sentence:
Examples:
- Never had she seen such a beautiful sunset.
- Rarely do we get such an opportunity.
- Not only did he win, but he also broke the record.
- Expressions of place.
Inversion sometimes happens with verbs of movement:
- On the hill stood a lonely cabin.
- Into the room walked the professor.
- Conditional structures (conditions)
Instead of using if , you can use formal inversion:
- Had I known, I would have helped.

Since ancient times, the Hebrew prophets have foretold the coming of a Messiah—a messenger from God who would bring salvation to His people. One of the most striking of these prophecies is found in Isaiah 53, which describes a “suffering servant” who would be rejected, punished for the transgressions of others, and through his suffering, bring healing and peace. Despite his apparent defeat, this servant would be exalted by God.
Centuries later, Christians see the life, death, and resurrection of Jesus Christ as the exact fulfillment of these words. Unjustly crucified, Jesus took on suffering for the sake of humanity and, when he rose again on the third day, was glorified, confirming his identity as the promised Messiah. For believers, his resurrection is proof that God’s ancient promises have been fully fulfilled.
For Christians, Jesus not only fulfilled Isaiah 53, but also several other messianic prophecies scattered throughout the Old Testament, such as in Psalms and Daniel. Easter, therefore, celebrates not only a historical event, but the fulfillment of a prophetic hope: the Messiah came, suffered, died and conquered death to offer eternal salvation to all who believe.

1. Basic Structure:
Question Word + Auxiliary Verb + Subject + Main Verb + Complement?
2. Questions with
-
-
3. Questions with
-
-
4. Questions with
-
-
5. Questions with
-
-
Attention! If
-
6. Questions with
-
7. Questions with
-

THE pulque is a traditional fermented beverage made from the juice extracted from the agave plant, which has been consumed by the indigenous peoples of Mexico for centuries. With its unique, slightly sour flavor, pulque has a rich history linked to the ancient Aztec and Toltec civilizations, who used agave to produce alcoholic beverages in religious ceremonies and celebrations.
Pulque production begins with the extraction of "miel de agave" (agave honey), which is naturally fermented to produce the beverage. Over time, pulque came to be consumed in a variety of ways, and during the colonial era it became a common drink among the working classes of Mexico, although it has lost popularity over the years in favor of more modern drinks such as tequila and mezcal.
Pulque was considered a sacred drink by the Aztecs and could only be consumed by priests, warriors or nobles. Its popularity has remained, especially in rural areas, and today it is consumed mainly in central and southern Mexico, often served in pulquerias, places that specialize in the drink.
Although it is not as common as it once was, pulque remains one of the most representative drinks of Mexican culture, connecting Mexicans with their historical and traditional roots.

THE Canadian whiskey It is widely recognized for its smoothness and elegance, winning admirers around the world. Its history dates back to the country's first settlers, who brought whisky distillation techniques from Europe. However, it was in Canada that whisky gained its own identity, characterized by its lightness and versatility, which makes it a perfect drink to be enjoyed neat, on the rocks or in cocktails.
Whisky production in Canada spread across the provinces, but it was in Ontario and Quebec that the first distilleries began producing this spirit commercially, in the 19th century. Over time, Canada has established itself as one of the largest whisky producers in the world.
Brands such as Crown Royal, which was created in 1939 to celebrate the visit of King George VI and Queen Elizabeth to Canada, are examples of how Canadian whisky has deep roots in the country's history. This particular whisky is known for its smooth blend, composed primarily of corn, which gives it a smoother, sweeter flavor than other types of whisky.
Today, Canadian whisky is a prestigious drink, widely enjoyed both in cocktails and in its pure form, and remains one of the country's most representative and respected alcoholic beverages.

The Casa Rosada, the seat of the Argentine government, is one of the most emblematic buildings in Buenos Aires. Its history dates back to the 16th century, when the site housed a Spanish fort to protect the city. Over time, the building was renovated and, in the 19th century, under the government of Domingo Faustino Sarmiento, it acquired its iconic pink color, which symbolized the union between the Unitarians (white) and Federalists (red), rival political factions at the time.
Located in Plaza de Mayo, the Casa Rosada has been the scene of historic events, such as speeches by Juan and Eva Perón, as well as notable political demonstrations. Today, in addition to being the center of executive power, the site houses the Casa Rosada Museum, where you can see presidential objects, antique furniture and exhibits on Argentine history.
Visitors can take free guided tours of the historic rooms, the famous presidential balcony and explore the palace's rich architecture. An essential tour for anyone wanting to understand Argentine politics and culture.

Opened in 1819, the Prado Museum (Museo del Prado) is one of the largest and most prestigious art museums in the world. Located in the heart of Madrid, Spain, it was founded by King Ferdinand VII to display the royal collections. The neoclassical building that houses it was designed by Juan de Villanueva and later expanded by other architects, such as Rafael Moneo.
With more than 8,000 works of art, the museum is famous for its vast collection of European painting, notably works by masters such as Diego Velázquez, Francisco Goya, El Greco, Peter Paul Rubens and Hieronymus Bosch. In addition to paintings, the Prado also houses sculptures, drawings and decorative arts.
Considered the main museum in Madrid and one of the most important in Europe, the Prado attracts millions of visitors annually. Its impressive collection and contribution to the preservation of classical art make the Prado Museum a must-see for any art lover visiting the city.

Catalan is the official language of Barcelona, and learning a few expressions can enrich any tourist's experience. Here are some useful phrases:
Bon dia – Good morning
Bona tarda – Good afternoon
Bona nit – Good evening
Com estàs? – How are you?
Molt bé, gràcies – Very well, thank you
On és el lavabo? – Where is the bathroom?
Què tal? – How are you?
Quant costa? – How much does it cost?
Tinc gana/sede – I'm hungry/thirsty
Un cafè si us plau– A coffee, please
Perdona, on està...? – Excuse me, where is...?
Si us plau – Please
Gràcies – Thank you
Adéu – Goodbye
Em pots ajudar? – Can you help me?
M'agrada – I like it
Although many in Barcelona speak Spanish, using Catalan is a sign of respect for the local culture, and the Barcelonans will certainly appreciate the effort.

Grammar is the set of rules and norms that govern the use of language. It defines how words are formed, combined and organized to express ideas clearly and efficiently. Grammar involves aspects such as morphology (the study of words), syntax (the ordering of words in sentences), semantics (the meaning of words) and phonology (the sounds of the language).
Studying grammar is essential to mastering a language, whether in written or spoken communication. By learning the grammatical rules, we are able to understand and produce texts correctly, coherently and precisely, in addition to avoiding ambiguities. Grammar also helps to preserve linguistic identity and facilitates the learning of other languages.
The first attempts to systematize grammar appeared in Antiquity, with scholars such as Plato and Aristotle, but it was with the Romans, such as Cicero, that the first more structured rules appeared. Modern grammar began to be formalized by scholars in the 16th century, such as Port-Royal, in France. Grammatical rules then emerged from the study and observation of languages over time, seeking patterns and norms to organize the use of language.
Biochemistry is the science that studies the chemical reactions and molecular processes that occur in living organisms. This interdisciplinary field combines principles of chemistry and biology to understand how cells work, including the structure and function of biomolecules such as proteins, carbohydrates, lipids and nucleic acids.
Biochemistry has fundamental applications in medicine, biotechnology, nutrition and pharmacology, enabling advances in the understanding of diseases, drug development and biomolecule engineering for various industrial sectors. Techniques such as genetic engineering and molecular biology drive research into metabolism, cell signalling and gene regulation.
In addition, biochemistry is essential for emerging fields, such as clinical biochemistry and industrial biotechnology, which seek innovative solutions for human health and sustainability. Its impact extends from the discovery of new treatments to the production of food and biofuels, making it an indispensable science for technological and scientific advancement.

The series "The Great Discovery" has become one of the biggest hits on television, captivating millions of viewers with its engaging and mysterious plot. With an average of 10 million viewers per episode, the series quickly became the top of the most-watched series.
The story follows an experienced detective who finds herself involved in a seemingly simple murder case in a small town. However, as the investigation progresses, deep secrets begin to be revealed, transforming the investigation into a web of mysteries much bigger than expected. The engaging narrative, combined with impactful performances and impeccable cinematography, made the series a ratings phenomenon.
Critics praised the well-structured script and complex characters, which guarantee unexpected twists in each episode. The success was so great that there is already speculation about a possible second season. "The Great Discovery" proves that the crime thriller genre continues to be one of the audience's favorites, combining tension, drama and mystery in an unforgettable production.

Jurassic World: Rebirth hits the big screen as one of the biggest productions in the franchise, maintaining the essence of action and adventure that has won over millions of fans around the world. The film, which is part of the sequel to the famous dinosaur series, brings a new and exciting story, expanding the Jurassic World universe with new threats, never-before-seen dinosaurs and a suspenseful plot.
With a plot that mixes nostalgia and innovation, the film follows the main characters trying to control a new generation of genetically modified dinosaurs. However, the situation gets out of control, unleashing chaos that puts humanity at risk. The script brings moments of breathtaking action and state-of-the-art special effects, which are a great attraction for fans of science fiction and entertainment cinema.
Jurassic World: Rebirth is a success in several parts of the world, breaking box office records and reaffirming the universal appeal of the franchise. With a strong director and cast, the film promises to become another milestone in the history of cinema, consolidating the popularity of prehistoric creatures among a new generation of viewers.
In 2019, the world was hit by an unprecedented pandemic caused by the SARS-CoV-2 coronavirus, which led to COVID-19, a highly contagious disease. The outbreak was initially detected in China and quickly spread across the globe, resulting in millions of infections and deaths. In March 2020, the World Health Organization (WHO) declared COVID-19 a pandemic.
The impact on public health was devastating. Healthcare systems in many countries were overwhelmed, with hospitals overwhelmed and essential equipment in short supply. The virus spread rapidly due to its transmissibility, forcing governments to implement strict social isolation and quarantine measures.
Economically, the pandemic caused a global recession, with a drop in production, business closures, rising unemployment and an unprecedented financial crisis. International trade was also severely affected, and many countries faced challenges in distributing effective vaccines and treatments.
Social interactions were profoundly altered, with physical distancing, mandatory mask-wearing and the transition to remote working and online learning. The pandemic has also highlighted social and economic inequalities, exacerbating mental health and well-being issues around the world.

Between the 16th and 17th centuries, the Scientific Revolution marked a radical change in human knowledge, challenging medieval beliefs and establishing the foundations of modern science. Great names such as Nicolaus Copernicus, Galileo Galilei and Isaac Newton revolutionized astronomy and physics, replacing old Aristotelian concepts with methods based on observation and experimentation.
In 1543, Copernicus published the heliocentric theory, stating that the Earth revolved around the Sun, contradicting the geocentric view defended by the Church. In the 17th century, Galileo, using the telescope, confirmed this theory and defied the Inquisition, being tried for heresy. Shortly thereafter, Newton formulated the laws of motion and gravity, cementing the scientific method and the mechanistic view of the universe.
The Scientific Revolution not only transformed astronomy and physics, but also influenced philosophy and technology, paving the way for the Enlightenment and the advances of modernity. This period redefined the relationship between science and religion and shaped the contemporary world, establishing reason as the pillar of human knowledge.

In 1453, the fall of Constantinople to the Ottomans, led by Sultan Mehmed II, marked the end of one of the oldest empires in history: the Byzantine Empire. The conquest of the city, a decisive milestone, profoundly altered the political and commercial dynamics of Europe.
With the weakening of the Byzantine Empire, the Ottomans established their dominance in the region and began to control important trade routes between the East and the West, impacting European trade. This led the European powers to seek new trade routes, stimulating the Age of Discovery and the great voyages of exploration, such as those of Christopher Columbus and Vasco da Gama, in an attempt to access the riches of the East.
In addition, the fall of Constantinople had significant cultural and intellectual implications. Many Byzantine scholars fled to the West, taking with them manuscripts and knowledge that contributed to the Renaissance, a period of great cultural, scientific and artistic effervescence in Europe.
This event not only marked the end of the Middle Ages, but also paved the way for the transition to the Modern Age, with great transformations in the fields of politics, culture and economy.

Homo floresiensis, popularly known as "the Hobbit", is one of the most intriguing discoveries in the study of human evolution. This species was identified in 2003 on the island of Flores, Indonesia, and surprised the scientific community due to its diminutive size and unique characteristics, challenging traditional conceptions of human evolution.
Standing only about 1 meter tall and with a remarkably small skull, Homo floresiensis lived until about 50,000 years ago, coexisting with modern humans. Its discovery has sparked debate about human origins and evolution, raising questions about the possibility that small groups of hominids survived longer than previously thought.
Homo floresiensis is believed to have been an isolated species, probably adapted to the specific environment of Flores, where geographic isolation would have led to the development of its distinctive physical characteristics, such as a small brain, which was similar in size to that of Australopithecus, a more ancient ancestor.
Homo floresiensis challenges conventional narratives of human evolution, suggesting that the story of our development may be more complex than previously thought.
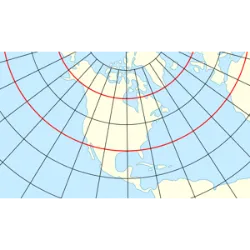
The Mercator Projection, one of the most widely used maps in the world, is not only a cartographic tool, but also carries a strong geopolitical meaning. Created in the 16th century by cartographer Gerardus Mercator to aid navigation, this projection preserves angles and directions, but distorts the real size of countries and continents. Regions close to the poles, such as Europe and North America, appear much larger than they actually are, while areas close to the equator, such as Africa and South America, are reduced.
This distortion reinforces historical inequalities, giving the impression that countries in the Northern Hemisphere are more important or dominant. For centuries, this view has influenced international politics, economics and even the cultural perception of nations. Alternatives such as the Gall-Peters Projection attempt to correct these inequalities by representing the continents in their real sizes, but they face resistance due to the consolidated use of the Mercator projection. The debate over maps and geopolitical power continues, showing that the way we see the world can influence the way we think about it.

Sports have been with humanity since ancient times. Records indicate that ancient civilizations, such as the Egyptians and Sumerians, already practiced physical activities for leisure and military training. In Ancient Greece, the Olympic Games, which began in 776 BC, consolidated sports competition, highlighting events such as running and wrestling. With the fall of the Roman Empire, sports lost ground, but were reborn in the Modern Age. In the 19th century, European countries standardized rules and created federations, promoting sports such as soccer and athletics. Today, sports are a global phenomenon, promoting health, culture and unity among nations.

In 2021, Jujutsu Kaisen not only won over audiences worldwide with its huge success, but also played a crucial role in popularizing the shonen genre among the younger generation in a new way. An interesting fact that many don't know is that the design of the protagonist Yuji Itadori was inspired by the style of the manga's author, Gege Akutami. The author drew Yuji with simpler, more "human" features, seeking to create a closer connection with the audience, making it easier for them to identify with the character.
In addition, Jujutsu Kaisen has stood out for an unexpected collaboration with the fashion world. Several streetwear brands have released collections inspired by the characters and iconic scenes from the series, showing how anime culture is increasingly influencing other industries, beyond animation. The growing popularity of anime reflects the evolution of how Japanese pop culture is interacting with fashion and other areas of contemporary society.

Cassim, a character from Aladdin and the Forty Thieves, is Aladdin's father and an accomplished leader of the thieves. Voiced by John Rhys-Davies, he left his family in search of a better life, but his wife died, leaving Aladdin an orphan. Cassim became the leader of the Forty Thieves and began a search for the Hand of Midas, an object capable of turning anything into gold.
In the film, Aladdin discovers that his father is alive and reunites with him. Despite attempts to reconcile, Cassim is arrested while trying to steal the royal treasure. Aladdin frees him, but refuses to run away with him. Cassim returns to the thieves, but is betrayed by Sa'Luk. In search of the Hand of Midas, father and son face dangers, until Cassim realizes the curse of the gold and discards the artifact, causing the destruction of the thieves. At the end, Cassim attends the wedding of Aladdin and Jasmine.

The final scene of The Little Mermaid (1989), where Ariel appears in a dazzling silver dress, is one of the most striking and technically challenging scenes in animation. The glitter effect on the dress was created entirely by hand, frame by frame, requiring meticulous work by the animators. At the time, digital techniques were not yet widely used, which made this scene an impressive feat.
Every inch of the dress was carefully lit to create a magical and ethereal effect, reinforcing the fairytale tone of the film. The soft glow of the fabric symbolizes Ariel's definitive transformation into a human and her new beginning with Prince Eric.
In addition to its technical complexity, the scene stands out visually, being considered one of the most beautiful in the film. The effort of the animation team resulted in an iconic moment that continues to enchant generations of fans. Ariel's silver dress became unforgettable and is still remembered today as one of Disney's most magical costumes.

Hans, a prince from a neighboring kingdom, initially appears as Anna's romantic interest in Frozen. After they quickly meet, he proposes to Anna, earning her trust. However, as the film progresses, he reveals himself to be a cunning and manipulative villain intent on taking the throne of Arendelle. His true nature emerges when he abandons Anna in danger and attempts to kill Elsa, shocking audiences and making him one of Disney's most memorable antagonists.
