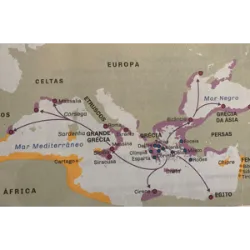
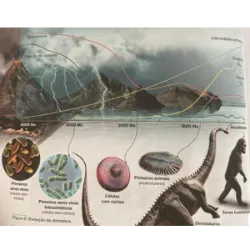
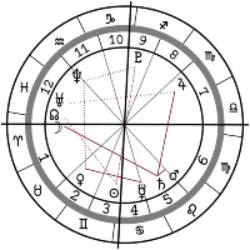
Curiosities, games, challenges and quiz on various topics
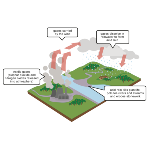
Acid rain is one of the consequences of air pollution. The carbon dioxide (CO2) existing in the atmosphere already makes rain slightly acidic, even under natural conditions. The natural pH of water is 7 and when in equilibrium with atmospheric CO2 it is 5.6, slightly acidic. Sulfur oxides (SO2 and SO3) and nitrogen oxides (N2O, NO and NO2) are the main components of acid rain. These compounds are released into the atmosphere through the burning of fossil fuels. When they react with water droplets in the atmosphere, they form sulfuric acid (H2SO4) and nitric acid (HNO3). Together, these two acids cause an increase in the acidity of rainwater. This acidification of soil and surface water has devastating effects on ecosystems and poses a serious danger to living beings.