Enzymology: Enzyme Kinetics
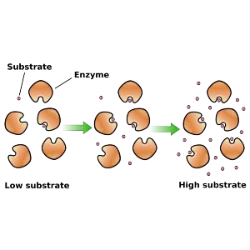
Enzymology studies enzymes and their mechanisms of action, which are fundamental to biochemical reactions in living organisms. Enzyme kinetics investigates how enzymes catalyze reactions and how variables influence their activity. The Michaelis-Menten model is the basis for understanding this process, describing the relationship between substrate concentration and the rate of the enzymatic reaction.
According to this model, the reaction rate increases with substrate concentration until it reaches a saturation point, when all enzymes are occupied. This point is represented by the maximum velocity (Vmax). The Michaelis constant (Km) indicates the affinity of the enzyme for the substrate; lower values of Km indicate greater affinity.
Several factors can affect enzyme activity, including temperature, pH, and the presence of inhibitors or activators. Increasing the temperature generally accelerates the reaction up to a certain limit, after which the enzyme may denature. The pH must also be adequate for the enzyme to maintain its active structure. Inhibitors can reduce enzyme activity, while activators increase it. Understanding these factors is essential for many areas of biotechnology and medicine.
Did you know?