pH is a measure of the acidity or alkalinity of an aqueous solution
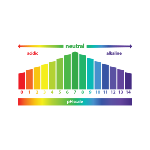
pH is a measure of the acidity or alkalinity of an aqueous solution, with values ranging from 0 to 14. 0 is the most acidic and 14 is the most alkaline, while 7 is neutral, having no effect on the system. The acronym "pH" stands for "hydrogen potential" and is used to measure the ratio of H3O+ to OH- ions in solution. According to the Arrhenius acid-base theory, acids are substances that release hydronium ions (H3O+) into water, and bases are substances that release hydroxyl ions (OH-). Water undergoes the autoionization process, which means that it contains the same amount of H3O+ and OH- in its composition, defining the pH of the solution.
Did you know?