The periodic table
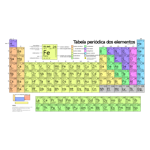
The periodic table is a system of classifying chemical elements by ordering them by atomic numbers, electron configurations, and periodic patterns of properties. This organization shows recurring trends, such as similar elements in the same columns. There are also four rectangular blocks that have similar chemical properties. As a rule, in the same line (period) the elements on the left are metallic and those on the right are non-metallic. These rows are called periods, while the columns are called groups. In addition to numbering, each group has a specific name, such as halogens (group 17) and noble gases (group 18). This table is used to understand relationships between element properties and to predict the characteristics of elements not yet discovered or produced. It provides a useful basis for understanding chemical behavior and is used extensively in chemistry and other areas of knowledge.
Did you know?